C is the specific heat of a material JgK. 131 Specific heat capacity.
For example the lower specific heat capacity of fat compared to other soft tissue indicates that fat requires.

. 009 Modulus of Elasticity. M is the mass of a material g. This means that it takes 4200 J to raise the temperature of one kg of water by 1 C.
Units of Heat - BTU Calorie and Joule - The most common units of heat BTU - British Thermal Unit Calorie and Joule. Dieter Haemmerich in Principles and Technologies for Electromagnetic Energy Based Therapies 2022. Material JkgK BtulbmF JkgC kJkgK Aluminium 887 0212 887 0887 Asphalt 915 021854 915 0915 Bone 440 0105 440 044 Boron 1106 0264 1106 1106 Brass 920.
Water - Specific Heat vs. LEADED RED BRASS CASTINGS SAND AND CENTRIFUGAL AS CAST. Specific Sub-Form Application System Standard Description.
T 2 T 1 is the temperature difference before and after heating or cooling K. WROUGHT AND CAST COPPER ALLOYS. A good website for this is peacesoftwarede the although we will need to convert the units to imperial so for that we will use Specific heat capacity and density of water.
Using the energy equation of Q ṁ x Cp x ΔT we can calculate the cooling capacity. Specific Heat Capacity Btu lb F at 68F. Substance Phase Isobaric mass heat capacity c P Jg 1 K 1 Molar heat capacity C Pm and C Vm Jmol 1 K 1 Isobaric volumetric heat capacity C Pv Jcm 3 K 1 Isochoric.
This will give us a specifi heat capacity of 10007643BTUlbF and density of 62414lbFt3. The specific heat capacity. Molar C Jmol K.
Table of specific heat capacities at 25 C 298 K unless otherwise noted. In thermodynamics and solid-state physics the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat Heat capacity in a solid. The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the.
Below this table is an image version for offline viewing. Citation needed Notable minima and maxima are shown in maroon. C in Jgm K.
The specific heat capacity c Jkg K of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K 1C. Temperature - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F - SI and Imperial units. Of water is 4200 joules per kilogram per degree Celsius JkgC.
The specific heat capacity of materials ranging from Water to Uranium has been listed below in alphabetical order. C in calgm K or Btulb F. Q is the heat absorbed or released by a material J.
It treats the vibrations of the atomic lattice heat as phonons in a box in contrast to the Einstein model which treats the solid as many individual non-interacting quantum harmonic.
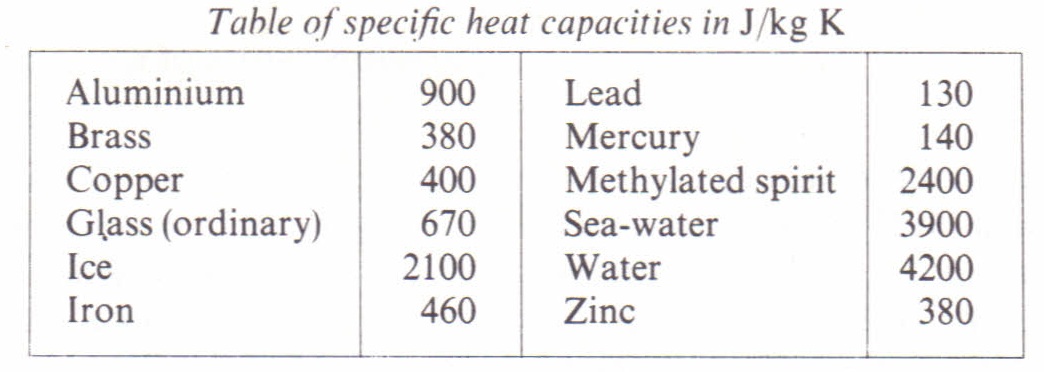
Specific Heat Capacity Physics Homework Help Physics Assignments And Projects Help Assignments Tutors Online

Which Metal Heats Up Fastest Aluminum Copper Or Silver Chemdemos

Can Anyone Suggest A Material With The Highest Specific Heat Capacity Higher Than Water

Solved Table 9 1 Specific Heat Capacity For Different Chegg Com
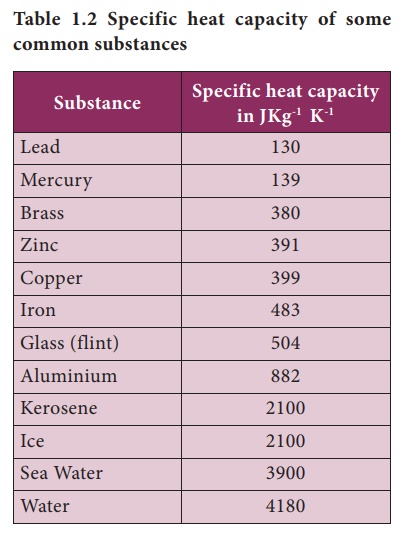
0 Comments